As we continue to explore top-scoring genes associated with the development of cancer through the Mapping Cancer Markers (MCM) project, we are excited to share a new research update! This article features NELL2 (Neural EGF-Like-Like protein 2), a gene that plays multiple roles in neural development and cell death regulation.
Terminology
- Glycoprotein: a protein in the body that has carbohydrates, or sugars, attached to the amino acids that make up its structure.
- EGF: Epidermal Growth Factor, a protein that is key to cell growth and differentiation.
- Homologue: in a genetic context, a homologue is a shared gene among two species that have a common ancestor.
- Biofluid: any kind of biological fluid produced by the body.
- RNA: ribonucleic acid, a molecule that is transcribed using a cell’s DNA, after which it can be used to create a protein when the RNA code is translated.
- Pathogenesis: the process through which disease develops, from initial cause to symptoms and tissue changes.
- Oncogenesis: the process through which normal cells turn into cancer cells.
- Contralateral: the side of the body opposite to where something originated.
- Isoform: a protein that has a similar biological function to another, but does not share the exact same amino acid sequence.
Project Background
MCM takes the remarkable computing power of the World Community Grid (WCG), supplied by a global community of over 817 000 volunteers, and uses it to screen millions of different data points collected from thousands of healthy and cancerous tissue samples. Through the systematic investigation of these data, we have identified 26 human genes that are strongly linked to the development of lung cancer. As of October 2025, MCM volunteer devices have generated over 2.77 billion results towards Mapping Cancer Markers. We sincerely thank you for your continued support, and appreciate your help in advancing humanitarian science.
Our last research update focused on ecdysoneless cell cycle regulator (ECD), a gene involved in cancer cell proliferation and tumour suppression. This update will describe NELL2, a gene crucial to the developing nervous system.
Introducing NELL2!
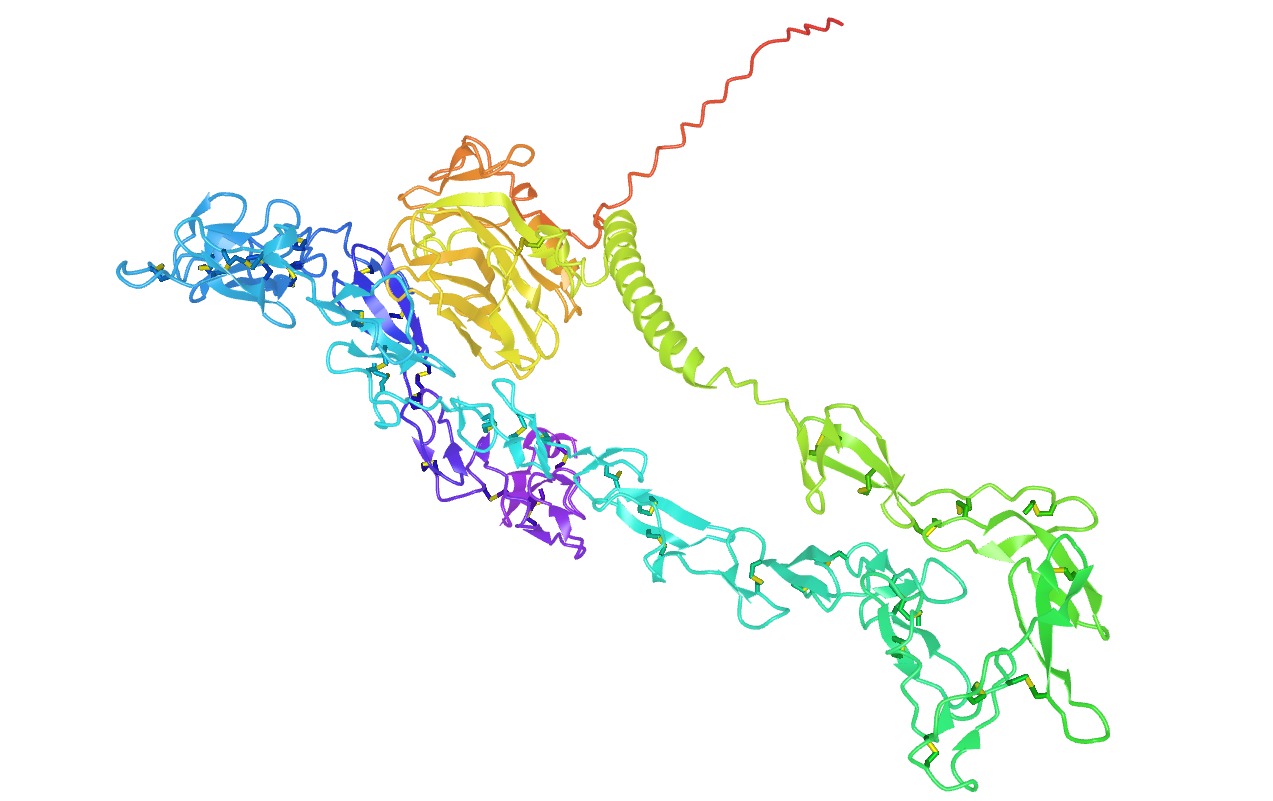
NELL2 is a gene that encodes the expression of a protein that cells secrete when the nervous system is developing (Fig. 1). This glycoprotein includes many EGF-like repeats that enable binding with calcium and proteins involved in the growth process (PMID: 32198364). NELL2 was discovered alongside NELL1 as a homologue of a chicken-derived protein (PMID: 8975702). After its discovery, the mammalian version of NELL2 turned out to exist in many other animal species as well, pointing to an evolutionary importance of its function.
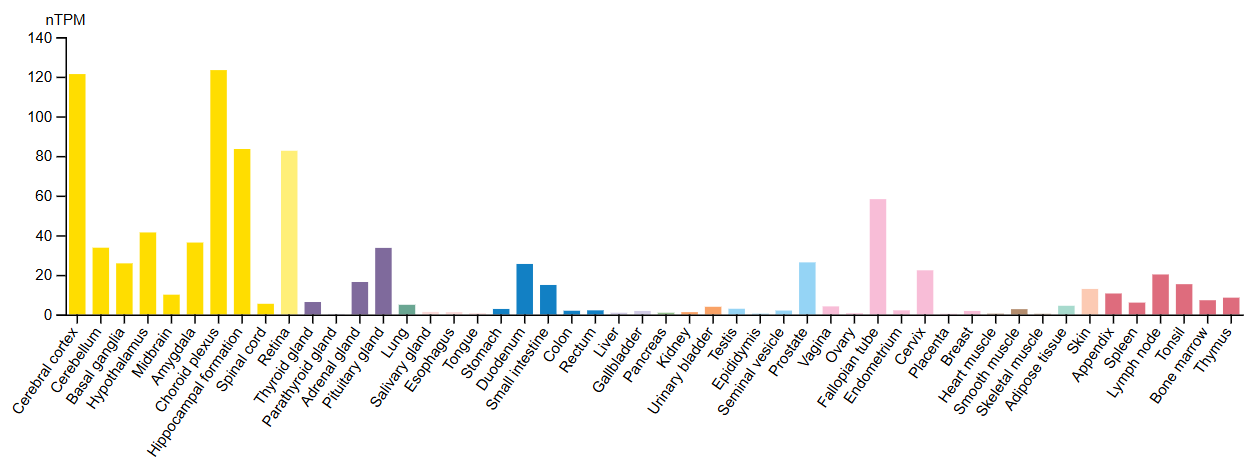
NELL2 is primarily found within the nervous system, but is also significantly expressed in reproductive tissue like the testis and fallopian tube (Fig. 2). This may be because of its role in sperm maturation and estrogen-dependent changes in the brain (PMID: 32499443, PMID: 20538601). Still, the bulk of NELL2 research is focused on the specific changes it triggers in the developing nervous system and how it triggers them. One possible method is through secretion, as NELL2 delivers information between cells after being secreted from those cells and travelling through various biofluids. This form of cell-to-cell communication makes it a valuable potential biomarker, as it could be detected through non-invasive methods, like blood tests.
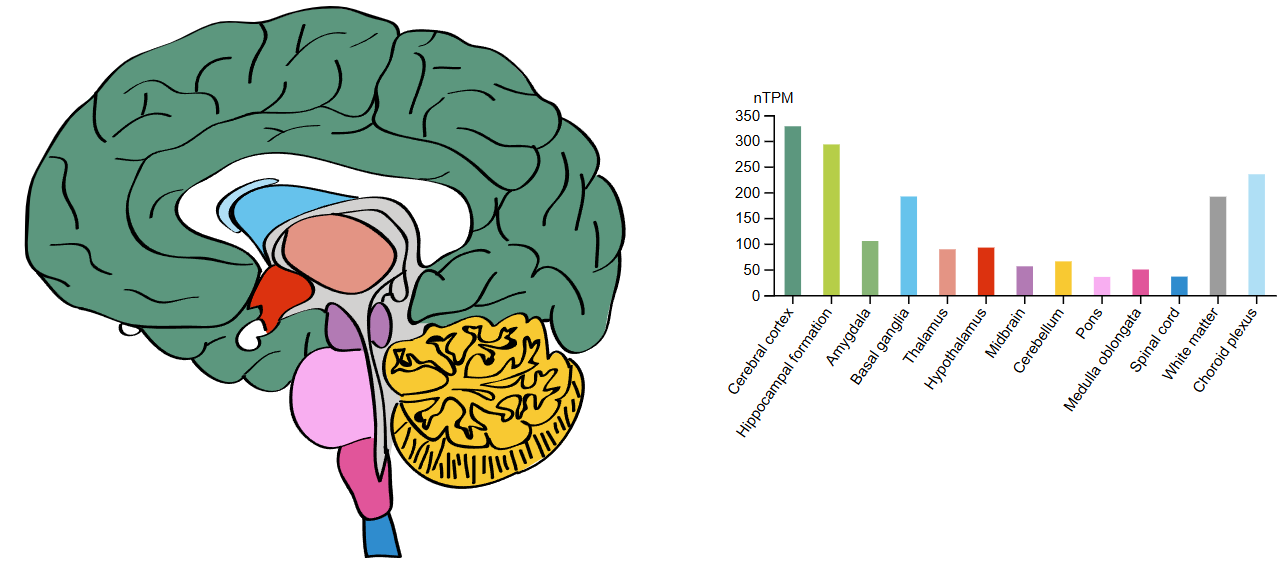
While NELL2 is widely expressed in the brain, it also features specific expression patterns within each region according to the different kinds of cells in the area (Fig. 3). For example, brain cells communicate with each other using signals that either activate or deactivate the next cell in their network, effectively passing on a message or preventing it from being passed on. The cells that pass on messages are excitatory, while the ones that prevent messages going through are inhibitory.
NELL2 expression in the cerebellum is restricted to brain cells that use inhibitory signalling, but is predominantly expressed in the excitatory brian cells of the hippocampus (PMID: 18677093). Though both types of brain cells exhibit completely opposing actions, NELL2 is most likely expressed in both due to their collective reliance on calcium to influence communication.
In addition to differentially regulated gene expression patterns, the NELL2 protein features many proposed mechanisms of action. The following section describes the key functions of NELL2 in the body and how it contributes to axonal guidance and neuron development, as well as its significance as a cancer marker.
Axonal guidance in the nervous system
Lung cancer has one of the highest cancer mortality rates in the world. This is due partly to its frequent metastasis to the brain, and is the source of approximately 50% of brain metastases (tumours from cancer that originated somewhere else). Because of this common path from lung cancer to brain metastasis, studying the pathogenesis of this phenomenon is an important part of understanding the disease as a whole (PMID: 28921309). Thus, we will explore the role of NELL2 in the nervous system as it develops to ensure an informed approach to the role of NELL2 in oncogenesis.
The spinal cord coordinates movement and sensation in the body through a large network of nerves. As an embryo develops, most of its nerves cross over the midline of the spinal cord and continue to project contralaterally en route to the brain (Fig. 4). This process is called decussation, and ensures that what is felt on the right side of your body is mostly processed by the left side of the brain. The part of the nerve that crosses over is the highway through which information travels: the axon. To properly establish these nerve fibre pathways and ensure that limbs and muscles are properly connected to the brain, axon growth is guided by many factors that influence length and direction (PMID: 24040928).
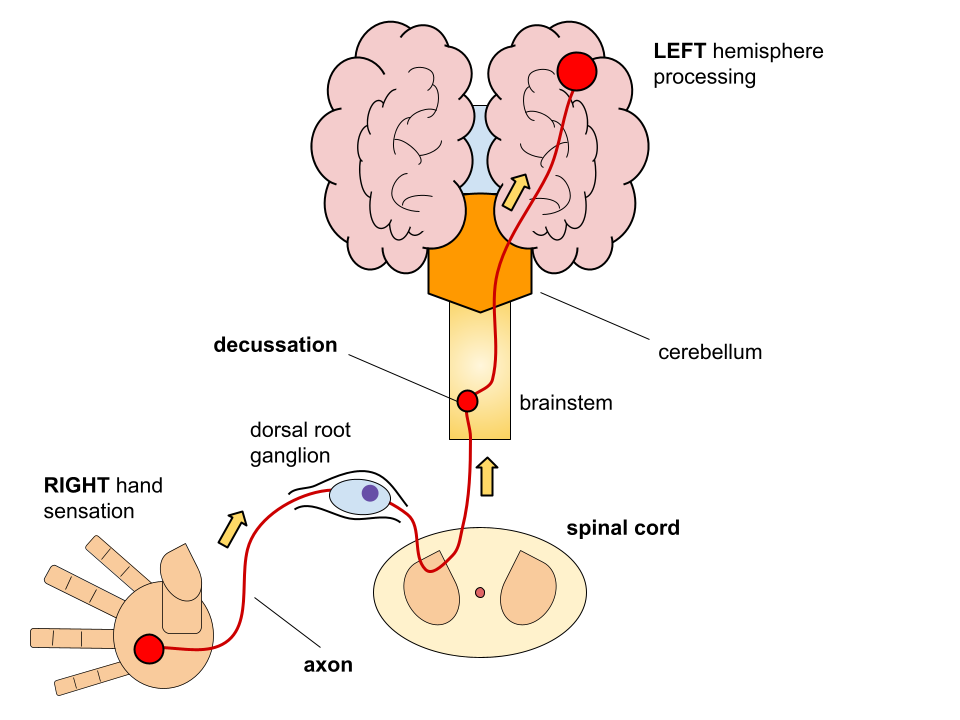
NELL2 binds to cell surface receptor Robo3 on axons to guide their journey to the contralateral side of the brain (PMID: 26586761). In this process, the axon expresses an isoform of Robo3 called Robo3.1 before crossing over. The reaction of NELL2 binding Robo3.1 guides the axon across the midline of the spinal cord, where it begins to change. Once across the midline, the axon starts to express a new receptor isoform called Robo3.2. NELL2 can no longer interact with this new protein, so the axon starts to grow straight again (PMID: 32198364). Thus, this system ensures that spinal cord neurons grow correctly and can send signals properly as the mammalian nervous system develops.
Beyond early neurodevelopment, NELL2 has also been linked to neuroblastoma and is highly expressed in cancerous tissue. As such, its involvement in a childhood cancer coupled with its role in nervous system development warrants further investigation into its function in neuroblastoma tumorigenesis (PMID: 11803583).
NELL2 in Lung Cancer
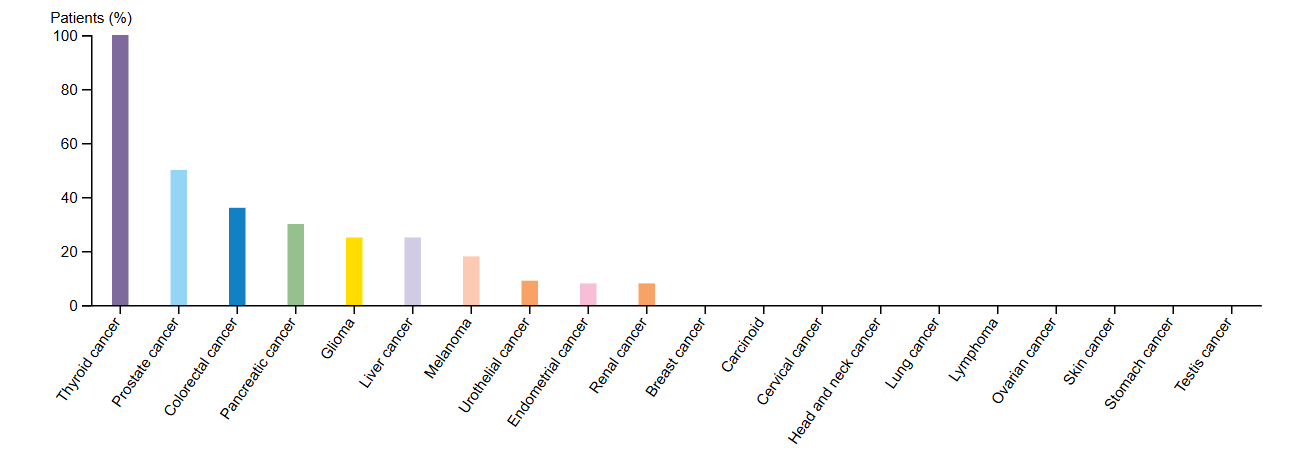
Lung cancer is one of the most frequently diagnosed cancers around the world. Its challenging diagnosis and poor prognosis make its treatment a significant issue for patients, but research continues to look for new ways to detect this disease. Tissue expression data points to significant NELL2 involvement in thyroid, prostate, colorectal, and pancreatic cancer (Fig. 5), yet despite showing limited expression in lung cancer, our recent findings identify it as a potential biomarker.
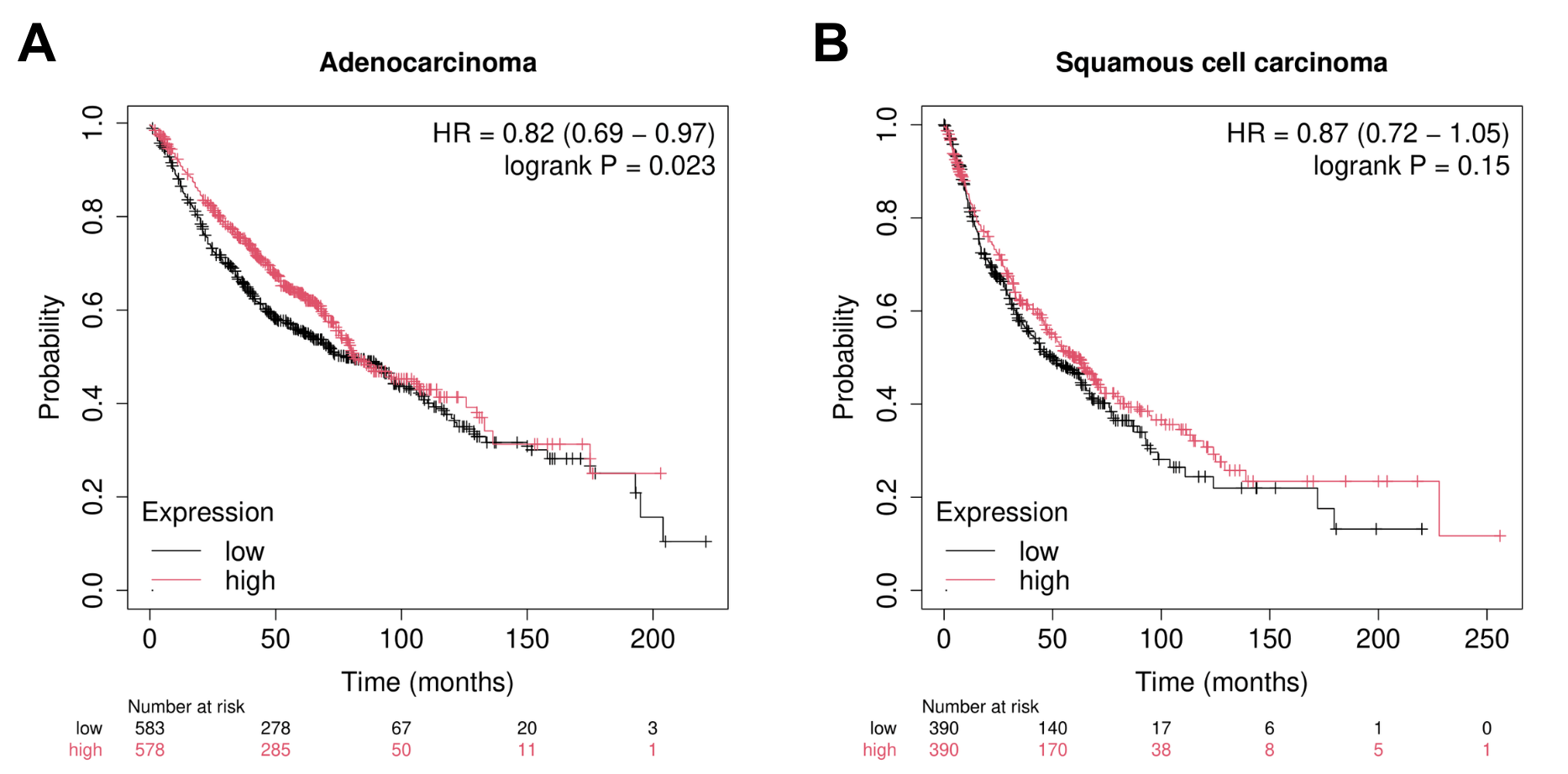
Adenocarcinoma (ADC) and squamous cell carcinoma (SQC) are the two most common subtypes of non-small cell lung cancer (NSCLC), together covering the majority of both NSCLC and lung cancer cases as a whole. While NELL2 has been primarily studied in cancers like renal cell carcinoma (RCC) and hepatocellular carcinoma (HCC), significant improvement in patient survival outcomes have been linked to high expression of NELL2 in lung ADC (Fig. 6A). However, these benefits do not seem to carry over to lung SQC (Fig. 6B).
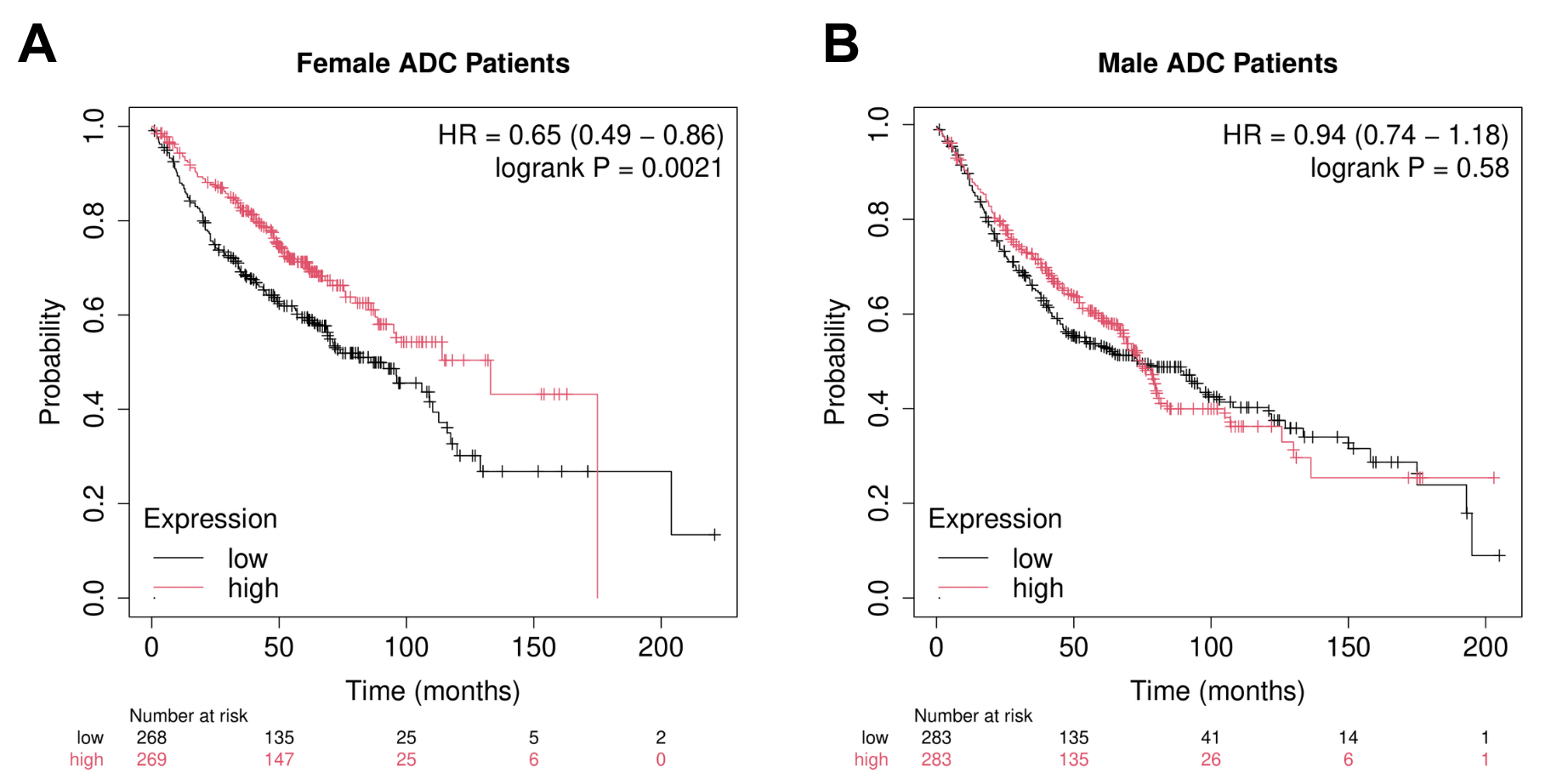
ADC arises from glandular cells (cells that produce and secrete fluids), whereas SQC originates from squamous cells that line the surface of the skin and other organs. These findings may indicate that NELL2 plays a more prominent role in glandular tumour biology rather than in squamous tumours due to its nature as a protein that is secreted through biofluids. This is also a strong indicator of its potential as a cancer biomarker since it can be detected non-invasively. However, the survival benefit of high NELL2 expression is only found in female ADC patients (Fig. 7A), while male ADC patient survival remains unaffected (Fig. 7B).
Further analyses into the effect of smoking on ADC or SQC in the context of NELL2 expression yielded no significant findings. Even so, the sex-dependent effect of NELL2 on survival raises interesting questions regarding NELL2’s interactions with sex-specific hormones and genetic factors influencing tumour biology. Prior studies have established a link between estrogen signalling and NELL2 transcription in the brain, as well as NELL2’s potential influence on sex-specific brain differentiation, but these links have yet to be explored in the context of lung cancer development (PMID: 20538601, PMID: 21643849).
Conclusion
NELL2 is a gene that influences multiple key processes in the body, from neural development and cell signaling to sperm maturation. Our analyses suggest that it also has a role in lung cancer, particularly in adenocarcinoma (ADC), where high expression correlates with improved survival in female patients. While its expression in squamous cell carcinoma (SQC) appears less impactful, the sex-dependent survival differences in ADC point to intriguing interactions between NELL2, hormones, and genetic factors that have yet to be fully explored. Altogether, these findings indicate exciting new ground to cover in the search for prognostic and diagnostic markers for lung cancer, with the MCM project at the forefront of this critical research.
Please reach out to us if you have any questions or comments regarding our research, or feel free to share your thoughts on the project forum.
Thank you for continuing to support the World Community Grid and empowering our research.
Sincerely,
The MCM Team